Quick-RNA 96 Kit
| Cat # | Name | Size |
| R1052 | Quick-RNA 96 Kit | 2 x 96 preps |
| R1053 | Quick-RNA 96 Kit | 4 x 96 preps |
Documents
Description
R1052 / R1053
Highlights
Broad Range: Extract total RNA (including small/micro RNAs) from any sample.
DNA-Free: Genomic DNA removal column and DNase I included.
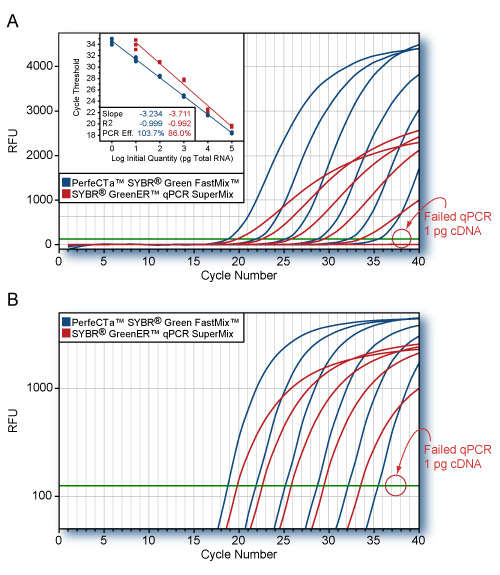
NGS-Ready: RNA is ready for all downstream applications including Next-Gen Sequencing, RT-qPCR, hybridization, etc.
Description
The Quick-RNA 96 Kit is one of the most innovative RNA isolation kits available, designed for the easy, reliable, and rapid isolation of DNA-free RNA from a wide range of cell (up to 106) and tissue samples (up to 5 mg). The procedure combines a unique buffer system with Zymo-Spin™ plate technology to yield high quality total RNA (including small RNAs ~17-200 nt) in about 30 minutes. The procedure is simple: Add the provided RNA Lysis Buffer to a sample, then purify the RNA using the provided Silicon-A Plate. The result is highly-concentrated, DNA-free RNA that is suitable for subsequent RNA-based methods including RT-PCR, hybridization, sequencing etc.
Equipment
Centrifuge/rotor compatible with 96-well plates
Purity
RNA is ready for Next-Gen sequencing, RTPCR, microarray, hybridization, etc. A260/A280, A260/A230: >1.8.
Sample Source
Cells or tissue samples, yeast, plant or bacteria. Compatible with DNA/RNA Shield and RNAlater.
Size Range
Total RNA ≥ 17 nt
Yield
10 µg RNA (binding capacity)
≥ 25 µl (elution volume)
Q1: What is the difference between the Quick-RNA Miniprep and the Quick-RNA Miniprep Plus?
Use the Quick-RNA Miniprep for cells and soft tissues. The “Plus” kit accommodates all sample types (cells, tissue, blood) and comes with DNA/RNA Shield (sample collection, transport, storage at ambient temperature).
Q2: How to store DNase-I following resuspension?
Lyophilized DNase I is stable at room temperature. Once resuspended, store frozen aliquots. Minimize freeze thaw cycles as much as possible. Freeze thaw will lower DNase activity.
Q3: Is the DNase-I treatment necessary?
If the downstream application requires DNA-free RNA, we recommend performing the DNase I treatment.
Q4: Is DNase I available for individual purchase?
Yes, the catalog number for the DNase I set (DNase and DNA Digestion Buffer) that we offer is E1010.
Q5: I ran out of RNA Wash Buffer. Can I use something else?
Yes. Use 80% ethanol as a substitute. RNA Wash Buffer is also sold separately.
Q6: Will the kit isolate small RNAs?
Yes, this kit will isolate small/micro RNA’s ≥ 17 nucleotides.
Q7: Can samples be stored in RNA Lysis Buffer prior to processing?
Yes, samples in RNA Lysis Buffer are stable overnight at room temperature and can be stored frozen (-80C). Be sure to lyse and homogenize the sample well prior to freezing. Bring the sample to room temperature prior to RNA Purification.
Q8: What is the difference between the Direct-zol RNA and Quick-RNA kits?
Direct-zol is for samples stored/collected into TRIzol/similar reagents. Quick-RNA is for all other samples.
Q9: Is it possible to extract proteins with the Quick-RNA kit?
Yes, proteins can be acetone precipitated from the column flowthrough. Please request supplementary protocol from Zymo Research Technical Support.
“Amazing results, the only RNA extraction kit I ever buy now. In my opinion, there is no product on the market that is as good a value and as effective as this kit for plant tissue RNA extractions.”
– Erin B. (Occidental College)
“Good price and easy to use, plus good quality. It is compatible to QIAGEN products RNeasy kit, but with reasonable price and equal quality. Especially the DNase is inexpensive and is included in the kit. No matter animal RNA, or plant RNA, or bacterial RNA, all could use with this one kit.”
– Xiaolu J. (Florida Atlantic University)
“I did an assay using RNA/DNA shield and it works perfect. The product have excellent reproducibility when stored at 4°C for up to a month.”
– Maha AE. (University of Florida)
“Easy and simple to use, even students can manage this! Reliable and in terms of value it is excellent. Color coded spin columns make it fool proof.”
– Jody H. (Otago University)