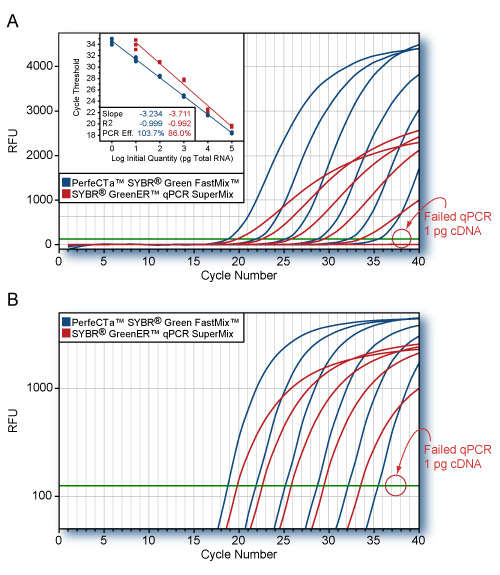
PerfeCTa SYBR Green FastMix comparison to SYBR GreenER qPCR SuperMix
RNA-specific adenosine deaminase (ADAR) was amplified from log-fold dilutions of total HeLa cell cDNA (100 ng to 1 pg) using PerfeCTa™ SYBR Green FastMix or the SYBR GreenER qPCR SuperMix (Invitrogen) according to each manufacturers protocol. Averaged plots for quadruplicate reactions for each input quantity are shown. Replicate CT values are shown on the standard curve (Panel A, inset). Cycling conditions: Invitrogenn: 95°C, 10 min followed by 40 cycles of 95°C, 10s; 60°C, 60s; PerfeCTa™ SYBR Green FastMix: 95°C, 20s followed by 40 cycles of 95°C, 1s; 60°C, 20s. PerfeCTa SYBR Green FastMix amplified the ADAR gene with higher efficiency and greater sensitivity. All replicate reactions for SYBR GreenER qPCR SuperMix failed to amplify ADAR from 1 pg of cDNA.