Trusted, accurate, and safe controls for a wide range of applications
The recent Coronavirus pandemic has led to the unprecedented need for diagnostic tests for detecting the presence of SARS-CoV-2 and other Respiratory viruses in a variety of sample types. To address this need, laboratories around the world need high quality tools to enable them in catering to this rapidly expanding requirement for testing.
Reproducible, Quality Results
Positive controls provide quality control measures for a wide range of applications from diagnostic assay development to day-to-day testing. This includes the verification and validation of diagnostic tests of both next-generation sequencing (NGS) and reverse transcription polymerase chain reaction (RT-PCR) assays.
A Safe and Effective Alternative to “Live Virus”
Controls based on viral nucleic acids extracted from either an infected patient or from live virus propagated in cell culture have safety and security concerns. Synthetic controls created through gene synthesis broaden access across diverse strains while mitigating risk.
Flexible formats for your applications
Choose from Standard, Assay Ready, or Encapsuled Control formulations. The standard format provides the controls at high concentration in a frozen liquid formulation. The Assay Ready Controls are provided as a dried pellet that increases stability. Just as protein encapsulation of viruses offers more stable molecular storage, Twist’s The Encapsulated Control products leverage a metal capsule sealed around a desiccated pellet to enhance stability of the RNA controls.
Both the Assay Ready and the Encapsulated formulations are single use controls. The Assay Ready format allows for shipping at ambient temperatures and storage in standard freezers. The enhanced stability of the Encapsulated controls enables extended shelf life and both ship and store at room temperature. Ambient shipping reduces cost and increases accessibility for researchers across the globe.
| Standard Controls | Assay Ready Controls | Encapsulated Controls | |
|---|---|---|---|
| Biosafety | Level 1 | Level 1 | Level 1 |
| Storage | Temperature: -70°C to -90°C | Temperature: -20°C | Ambient (4°C - 40°C) |
| Specification Range | Approximately 1x10^6 copies/μL | Approximately 2x10^6 copies/tube | Approximately 50,000 copies/tube |
| Physical State | Frozen liquid | Dried pellet | Encapsulated dried pellet |
| Shelf Life | 2 years | 2 years | 5 years |
| Shipping | Dry Ice | Ambient | Ambient |
KEY BENEFITS

- Includes fully synthetic RNA generated from Twist gene fragments
- Powerful alternative to live viruses

- >99.9% viral genome coverage
- NGS sequence verified
- Enables versatility in assay design

- Positive controls perform for both qPCR and NGS assays
- Content is well-aligned with Twist target enrichment panels
Monkeypox Controls
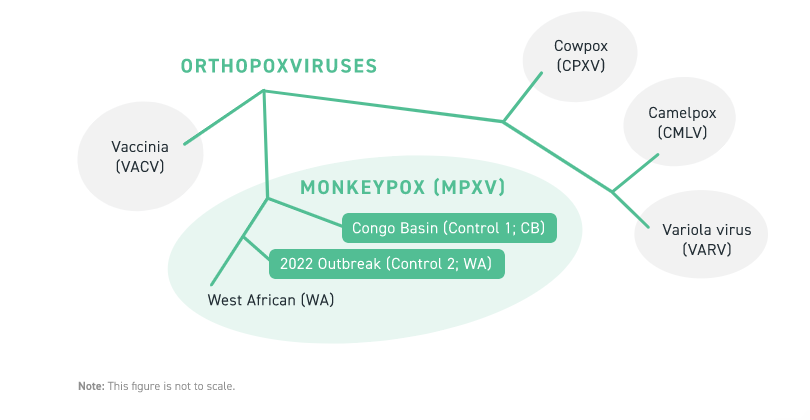
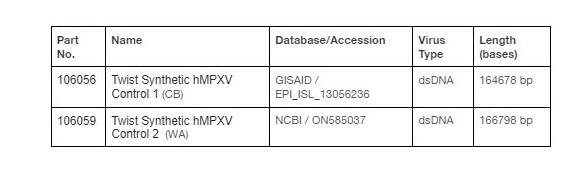
Twist’s Synthetic human Monkeypox Virus (hMPXV) Controls are based on sequences from either the Congo Basin (CB) or West African (WA) clades of hMPXV and provide coverage of ≥80% of the viral genome. As a result, these controls support the design of custom assays targeting regions of the genome relevant to orthopoxvirus research and can be used for both amplicon and capture-based detection methods. Twist’s hMPXV controls have been validated and are compatible with CDC recommended real-time PCR testing procedures for monkeypox detection. Control 1 and Control 2 are composed of 154 and 151 overlapping 1.8kb fragments, respectively, which tile the genome at an interval of 900bp. Each control is present at copies and provided at a concentration of approximately 100,000 copies/uL. The figure on the right shows a taxonomic relationship between the WA and CB clades of hMPXV as well as other orthopoxviruses. Please contact your local sales representative for more information.
Note: The Twist Synthetic human Monkeypox Virus
(hMPXV) Controls are not ISO-13485 certified
The use of positive controls in quality control
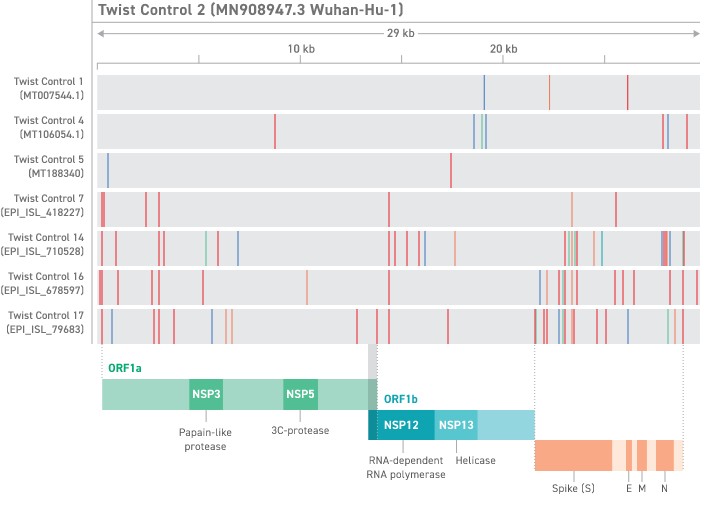
Twist has created synthetic RNA controls for several viral genomes. These variants were selected to cover a wide range of sequence diversity. Each control consists of six non-overlapping 5 kb fragments generated from Twist Gene Fragments then transcribed into ssRNA. These controls provide coverage of greater than 99.9% of the bases of the SARS-CoV-2 viral genome. The majority of the controls are provided in the standard format which consist of 100 μL at a concentration of approximately one million copies per microliter. Twist also offers certain controls in Assay Ready and Encapsulated Formats that are supplied as a desiccated pellet. The Assay Ready Controls are supplied at approximately two million copies per tube. The Encapsulated Controls are supplied at approximately 50,000 copies per tube. The Twist Assay Ready Synthetic SARS-CoV-2 RNA Control was developed in partnership with the US CDC and integrated into the CDC Influenza SARS-CoV-2 (Flu SC2) Multiplex Assay, which tests for influenza A, B and SARS-CoV-2 simultaneously. The Twist Assay Ready Synthetic SARS-CoV-2 RNA Control is now available for customers outside the CDC.
Twist Bioscience Encapsulated Control products offer a unique storage and delivery method for Twist’s established Synthetic SARS-CoV-2 RNA controls. Just as protein encapsulation of viruses offers more stable molecular storage, Twist’s Encapsulated Control products leverage a novel method to seal a metal capsule around a desiccated pellet to enhance stability of the RNA controls. Due to the enhanced stability, they can be shipped and stored at room temperature, reducing cost and improving accessibility globally. The Encapsulated Controls are sold in a rack of 16 or 96 tubes, are single use, and have a 5-year shelf life from the date of manufacture.
This product has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories; This product has been authorized only for the detection of nucleic acid from SARS-CoV-2, along with the identification and differentiation of SARS-CoV-2 PANGO lineages and the identification of specific SARS-CoV-2 genomic mutations, not for any other viruses or pathogens; and, the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
| PART # | CONTROL | GenBank/GISAID ID | GISAID NAME |
| 102019 | Control 1 | MT007544.1 | Australia/VIC01/2020 |
| 102024 | Control 2 | MN908947.3 | Wuhan-Hu-1 |
| 103925 | Assay Ready Control 2 | MN908947.3 | Wuhan-Hu-1 |
| 104422 | Encapsulated Control 2 (16) | MN908947.3 | Wuhan-Hu-1 |
| 104426 | Encapsulated Control 2 (96) | MN908947.3 | Wuhan-Hu-1 |
| 102860 | Control 3 | LC528232.1 | Japan/Hu_DP_Kng_19-020/2020 |
| 102862 | Control 4 | MT106054.1 | USA/TX1/2020 |
| 102917 | Control 5 | MT188340 | USA/MN2-MDH2/2020 |
| 102918 | Control 6 | MT118835 | USA/CA9/2020 |
| 103087 | Control 7 | EPI_ISL_418227 | France/HF2393/2020 |
| 103511 | Control 8 | MT066176 | Taiwan/NTU02/2020 |
| 103512 | Control 9 | MT152824 | USA/WA2/2020 |
| 103513 | Control 10 | EPI_ISL_414648 | USA/CA-PC101P/2020 |
| 103514 | Control 11 | EPI_ISL_417739 | Iceland/5/2020 |
| 103515 | Control 12 | EPI_ISL_420244 | England/SHEF-C05B2/2020 |
| 103533 | Control 13 | EPI_ISL_421184 | Belgium/ULG-10004/2020 |
| 103907 | Control 14 Alpha |
EPI_ISL_710528 | England/205041766/2020 |
| 103926 | Assay Ready Control 14 Alpha (B.1.1.7) |
EPI_ISL_710528 | England/205041766/2020 |
| 103909 | Control 15 Alpha |
EPI_ISL_601443 | England/MILK-9E05B3/2020 |
| 103927 | Assay Ready Control 15 Alpha (B.1.1.7) |
EPI_ISL_601443 | England/MILK-9E05B3/2020 |
| 104043 | Control 16 Beta (B.1.351) |
EPI_ISL_678597 | South Africa/KRISP-EC-K005299/2020 |
| 104044 | Control 17 Gamma (P.1) |
EPI_ISL_792683 | Japan (Brazil) /IC-0564/2021 |
| 104338 | Control 18 Kappa (B.1.617.1) |
EPI_ISL_1662307 | India/CT-ILSGS00361/2021 |
| 104529 | Control 19 Iota (B.1.526) |
EPI_ISL_1300881 | USA/NY-MSHSPSP-PV24650/2020 |
| 104530 | Control 20 (B.1.427) | EPI_ISL_730092 | USA/CA-ALSR-4704/2020 |
| 104531 | Control 21 Epsilon (B.1.429) |
EPI_ISL_672365 | USA/CA-CZB-12943/2020 |
| 104532 | Control 22 (B1.1.519) | EPI_ISL_933685 | Mexico/CMX-InDRE_208/2021 |
| 104533 | Control 23 Delta (B.1.617.2) |
EPI_ISL_1544014 | India/MH-NCCS-P1162000182735/2021 |
| 104534 | Control 24 (B.1.617.3) |
EPI_ISL_1939891 | India/MH-SEQ-221_S66_R1_001/2021 |
| 104538 | Control 28 Delta (AY.1) |
EPI_ISL_2695467 | Portugal/PT9543/2021 |
| 104423 | Encapsulated Control 28 (16) Delta (AY.1) | EPI_ISL_2695467 | Portugal/PT9543/2021 |
| 104427 | Encapsulated Control 28 (96) Delta (AY.1) | EPI_ISL_2695467 | Portugal/PT9543/2021 |
| 104539 | Control 29 Delta (AY.2) |
EPI_ISL_2693246 | USA/WA-CDC-UW21061750277/2021 |
| 105204 | Control 48 (B.1.1.529/BA.1) | EPI_ISL_6841980 | Hong Kong/HKU-211129-001/2021 |
| 104424 | Encapsulated Control 48 (16) (B.1.1.529/BA.1) |
EPI_ISL_6841980 | Hong Kong/HKU-211129-001/2021 |
| 104428 | Encapsulated Control 48 (96) (B.1.1.529/BA.1) |
EPI_ISL_6841980 | Hong Kong/HKU-211129-001/2021 |
| 105345 | Control 50 (B.1.1.529+BA.2) | EPI_ISL_7190366 | Australia/QLD2568/2021 |
| 105346 | Control 51 (B.1.1.529+BA.2) | EPI_ISL_7718520 | England/MILK-2DF642C/2021 |
| 105865 | Control 62 (B.2.12.1) | EPI_ISL_12248637.1 | Denmark/DCGC-493190/2022 |
| 105857 | Control 63 (B.2.12.1) | EPI_ISL_12303256.1 | USA/NY-CDC-LC0579415/2022 |
| 106196 | Control 64 (BA.5) | EPI_ISL_12516495 | hCoV-19/England/LSPA-3DC1269/2022 |
| 106197 | Control 65 (BA.5) | EPI_ISL_12620611 | hCoV-19/USA/TN-ASC-210769476/2022 |
| 106198 | Control 66 (BA.4) | EPI_ISL_12454576 | hCoV-19/USA/TX-HMH-M-96682/2022 |
| 106199 | Control 67 (BA.4) | EPI_ISL_12605687 | hCoV-19/USA/CA-CDC-QDX36065390/202 |
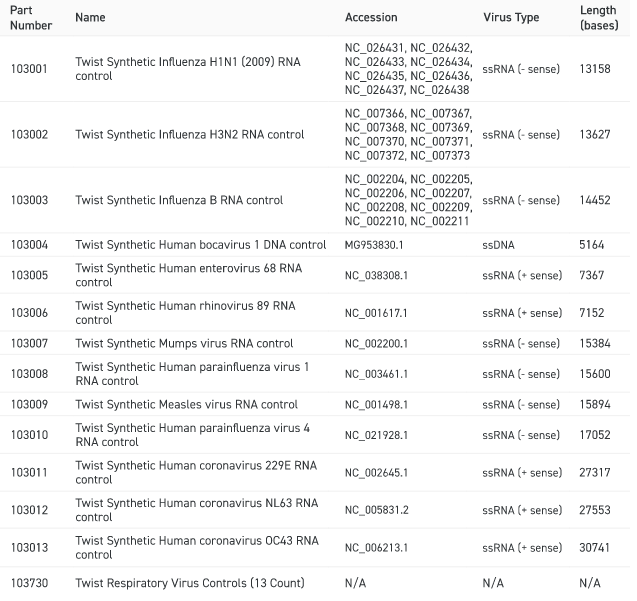
Available Respiratory Virus Controls
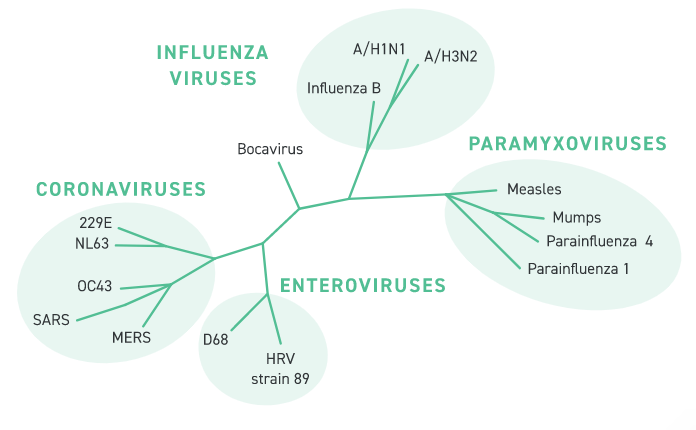
The Twist Synthetic Respiratory Virus Controls cover a broad range of RNA and DNA viruses relevant to respiratory disease research. These controls are aligned with the content of the Twist Respiratory Virus Research Panel (PN 103067) and are suitable for use with Twist Fixed Panel NGS workflows or for use with RT-PCR or qPCR experiments of your own design.
The figure shows a taxonomic tree of viruses covered by the Twist Respiratory Virus Controls. The table shows GenBank IDs, virus type and length for each control. Additional viral controls may be available for custom synthesis. Please contact your local sales representative for more details.
PRODUCT SHEET
- Synthetic SARS-CoV-2 RNA Controls
- Respiratory Virus Controls
- Twist Synthetic Infectious Disease Controls Product List
- Synthetic SARS-CoV-2 Control Variant Information
TECHNICAL NOTE
TECHNICAL DOCUMENT