Perfecta MultiPlex qPCR Super Mix
| Products | Kit Size | Order Info |
|---|---|---|
| PerfeCTa MultiPlex qPCR SuperMix | 200 x 50 μL rxns (4 x 1.25 mL) | Order (95063-200) |
| 1000 x 50 μL rxns (1 x 25 mL) | Order (95063-01K) | |
| PerfeCTa MultiPlex qPCR SuperMix Low ROX | 200 x 50 μL rxns (4 x 1.25 mL) | Order (95108-200) |
| 1000 x 50 μL rxns (1 x 25 mL) | Order (95108-01K) |
Documents
Description
Sensitive and robust multiplex qPCR assay performance with user-friendly 1-tube reagents
Features & Benefits
- Available in two versions: Universal and Low-ROX
- Maximum assay sensitivity and target precision with stringent, ultrapure, and high-yielding AccuStart II hot start Taq DNA polymerase
- Broad linear detection range with cDNA or genomic DNA templates
- Enables highly sensitive 4 target detection assays with coamplified, robust internal positive controls
- Supports efficient vortex mixing with proprietary anti-foaming technology
PerfeCTa Multiplex qPCR SuperMix is intended for molecular biology applications. This product is not intended for the diagnosis, prevention or treatment of a disease.
Description
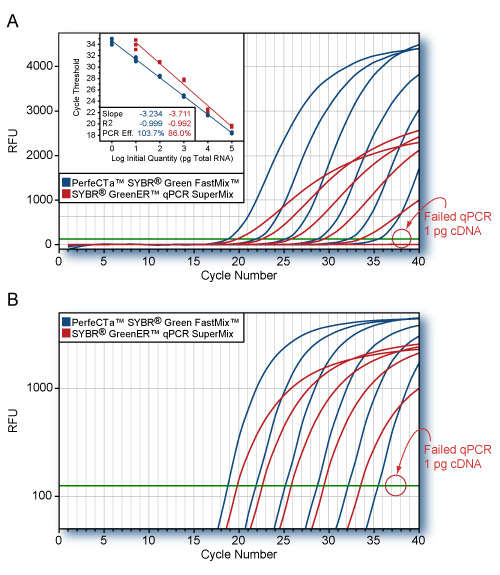
PerfeCTa Multiplex qPCR SuperMix is a 2X concentrated, ready-to-use reaction cocktail that contains all the necessary components except: primers, probe(s), and DNA template for highly-multiplexed, real-time quantitative PCR. This reagent formulation pushes the boundary of multiplex qPCR by enabling unbiased amplification of up to 5 targets in a single amplification reaction. Suppression of low abundance targets by high abundance reference targets during co-amplification is a common problem in multiplex PCR in which individual assay sensitivity can be significantly compromised. PerfeCTa Multiplex qPCR SuperMix, Low ROX delivers assay performance with exceptionally broad, linear detection and limit-of-detection (LOD) sensitivity to multiplexed qPCR that is comparable single-plex assay performance without the need for rigorous titration of individual primer assays. A key component of this SuperMix is ultra-pure AccuStart™ hot start Taq DNA polymerase that is completely arrested prior to the initial PCR denaturation step. Upon heat activation at 95°C, the antibodies are rapidly and irreversibly denatured, releasing a fully active high-yielding Taq DNA polymerase mutant. This enables specific and efficient primer extension with the convenience of ambient room-temperature reaction assembly.
Store components in a constant temperature freezer at -25°C to -15°C protected from light upon receipt.
For lot specific expiry date, refer to package label, Certificate of Analysis or Product Specification Form.
Publications
Ability of device to collect bacteria from cough aerosols generated by adults with cystic fibrosis
David N. Ku, F1000 Research – 2016
ABSTRACT
Background: Identifying lung pathogens and acute spikes in lung counts remain a challenge in the treatment of patients with cystic fibrosis (CF). Bacteria from the deep lung may be sampled from aerosols produced during coughing. Methods: A new device was used to collect and measure bacteria levels from cough aerosols of patients with CF. Sputum and oral specimens were also collected and measured for comparison. Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, and Streptococcus mitis were detected in specimens using Real-Time Polymerase Chain Reaction (RT-PCR) molecular assays. Results: Twenty adult patients with CF and 10 healthy controls participated. CF related bacteria (CFRB) were detected in 13/20 (65%) cough specimens versus 15/15 (100%) sputum specimens. Commensal S. mitis was present in 0/17 (0%, p=0.0002) cough specimens and 13/14 (93%) sputum samples. In normal controls, no bacteria were collected in cough specimens but 4/10 (40%) oral specimens were positive for CFRB. Conclusions: Non-invasive cough aerosol collection may detect lower respiratory pathogens in CF patients, with similar specificity and sensitivity to rates detected by BAL, without contamination by oral CFRB or commensal bacteria.
Parvovirus B19 integration into human CD36+ erythroid progenitor cells
Tyler Janovitz, Virology – 2017
ABSTRACT
The pathogenic autonomous human parvovirus B19 (B19V) productively infects erythroid progenitor cells (EPCs). Functional similarities between B19V nonstructural protein (NS1), a DNA binding endonuclease, and the Rep proteins of Adeno-Associated Virus (AAV) led us to hypothesize that NS1 may facilitate targeted nicking of the human genome and B19 vDNA integration. We adapted an integration capture sequencing protocol (IC-Seq) to screen B19V infected human CD36+ EPCs for viral integrants, and discovered 40,000 unique B19V integration events distributed throughout the human genome. Computational analysis of integration patterns revealed strong correlations with gene intronic regions, H3K9me3 sites, and the identification of 41 base pair consensus sequence with an octanucleotide core motif. The octanucleotide core has homology to a single region of B19V, adjacent to the P6 promoter TATA box. We present the first direct evidence that B19V infection of erythroid progenitor cells disrupts the human genome and facilitates viral DNA integration.
Torunn Gresdal Rønning, Acta Paediatrica – 2018
ABSTRACT
Aim Klebsiella spp. have been stated to be the most frequent cause of neonatal intensive care unit (NICU) outbreaks. We report an outbreak of Klebsiella oxytoca in a NICU at a tertiary care hospital in Norway between April 2016 and April 2017. This study describes the outbreak, infection control measures undertaken and the molecular methods developed. Methods The outbreak prompted detailed epidemiological and microbial investigations, where whole-genome sequencing (WGS) was particularly useful for both genotyping and development of two new K. oxytoca-specific real-time PCR assays. Routine screening of patients, as well as sampling from numerous environmental sites, was performed during the outbreak. A bundle of infection control measures was instigated to control the outbreak, among them strict cohort isolation. Results Five neonates had symptomatic infection, and 17 were found to be asymptomatically colonised. Infections varied in severity from conjunctivitis to a fatal case of pneumonia. A source of the outbreak could not be determined. Conclusion This report describes K. oxytoca as a significant pathogen in a NICU outbreak setting and highlights the importance of developing appropriate microbiological screening methods and implementing strict infection control measures to control the outbreak in a setting where the source could not be identified.
RAPID GENE EXPRESSION BASED DOSE ESTIMATION FOR RADIOLOGICAL EMERGENCIES
Stanislav Polozov, Radiation Protection Dosimetry – 2019
ABSTRACT
Gene expression (GE) assays have shown great potential for rapid individual radiation dose exposure assessment. The aim of the present study was to optimise GE-based biological dosimetry protocols for radiological emergencies. Experiments were carried out to validate a newly developed protocol (P2) where several steps were optimised and to compare it with the current validated protocol in place in our laboratory (P1). Several donor blood samples from were exposed ex vivo to of the following doses: 0, 0.5, 1, 2 Gy X-rays. Concomitant measurement of transcription level of genes FDXR, P21, PHPT1, CCNG1 and SESN1 plus HPRT (control) was performed. To summarise, both protocols provided similar dose estimates, P1 being completed in 7 hours while P2 in merely 4 hours. Thus, a significant time shortening was achieved leading to a potential increase of throughput capacity. Hence, this new protocol can be recommended for mass radiation casualties triage purposes.
Torunn Gresdal Ronning, Acta Paediatrica – 2019
ABSTRACT
Aim Klebsiella spp. have been stated to be the most frequent cause of neonatal intensive care unit (NICU) outbreaks. We report an outbreak of Klebsiella oxytoca in a NICU at a tertiary care hospital in Norway between April 2016 and April 2017. This study describes the outbreak, infection control measures undertaken and the molecular methods developed. Methods The outbreak prompted detailed epidemiological and microbial investigations, where whole‐genome sequencing (WGS) was particularly useful for both genotyping and development of two new K. oxytoca‐specific real‐time PCR assays. Routine screening of patients, as well as sampling from numerous environmental sites, was performed during the outbreak. A bundle of infection control measures was instigated to control the outbreak, among them strict cohort isolation. Results Five neonates had symptomatic infection, and 17 were found to be asymptomatically colonised. Infections varied in severity from conjunctivitis to a fatal case of pneumonia. A source of the outbreak could not be determined. Conclusion This report describes K. oxytoca as a significant pathogen in a NICU outbreak setting and highlights the importance of developing appropriate microbiological screening methods and implementing strict infection control measures to control the outbreak in a setting where the source could not be identified.
Safety Data Sheets (SDS)
CofA (PSF)
PSF-95063-200-Lot#016313
PSF-95108-01K-Lot#013958
PSF-95108-01K-Lot#015903
PSF-95108-200-Lot#016454
PSF-95108-200-Lot#019105
PSF-95063-01K-Lot#021959
PSF-95063-01K-Lot#023118
PSF-95063-01K-Lot#023119
PSF-95063-01K-Lot#023120
PSF-95063-200-Lot#021751
PSF-95063-200-Lot#021752
PSF-95063-200-Lot#021945
PSF-95063-200-Lot#022028
PSF-95063-200-Lot#022041
PSF-95063-200-Lot#022337
PSF-95063-200-Lot#022529
PSF-95063-200-Lot#022530
PSF-95063-200-Lot#022531
PSF-95108-200-Lot#020782
PSF-95108-200-Lot#021534
PSF-95108-200-Lot#022049
PSF-95108-01k-Lot#021528
PSF-95108-01K-Lot#023670
PSF-95108-200-Lot#023904
PSF-95108-200-Lot#023904
PSF-95108-01K-Lot#025040
PSF-95063-050-Lot#025123
PSF-95063-01K-Lot#025253
PSF-95108-01K-Lot#026328
PSF-95063-200-Lot#026805
PSF-95063-200-Lot#027077
PSF-95108-01K-Lot#027188
PSF-95063-01K-Lot#027318
PSF-95108-01K-Lot#027633
PSF-95063-050-Lot#027981
PSF-95063-200-Lot#028139
PSF-95063-01K-Lot#028141
PSF-95108-050-Lot#028460
PSF-95108-050-Lot#029135
PSF-95063-01K-Lot#029294
PSF-95063-050-Lot#029392
PSF-95108-050-Lot#029912
PSF-95108-01K-Lot#030090
PSF-95108-200-Lot#023904
PSF-95063-050-LOT#66139493
PSF-95063-01K-LOT#66141021
PSF-95063-200-LOT#66146451
PSF-95063-200-LOT#66146500
PSF-95108-050-LOT#66147789
PSF-95063-050-LOT#66148491
PSF-95108-200-LOT#66146569
PSF-95108-01K-LOT#66153069
PSF-95108-01K-LOT#66154458
PSF-95063-01K-LOT#66153739
PSF-95063-050-LOT#66154987
PSF-95063-200-LOT#66154988
PSF-95108-200-LOT#66157573