UltraPlex 1-Step ToughMix
| Product | Kit Size | Order Info |
|---|---|---|
| UltraPlex 1-Step ToughMix | 100 x 20 µL rxns (1 x 500 µL) | Order (95166-100) |
| 500 x 20 µL rxns (5 x 500 µL) | Order (95166-500) | |
| 1000 x 20 µL rxns (1 x 5 mL) | Order (95166-01K) | |
| UltraPlex 1-Step ToughMix ROX | 100 x 20 µL rxns (1 x 500 µL) | Order (95167-100) |
| UltraPlex 1-Step ToughMix Low ROX | 100 x 20 µL rxns (1 x 500 µL) | Order (95168-100) |
| 500 x 20 µL rxns (5 x 500 µL) | Order (95168-500) |
Documents
Description
Tough-tested, multiplex, 1-step real time PCR
Features & Benefits
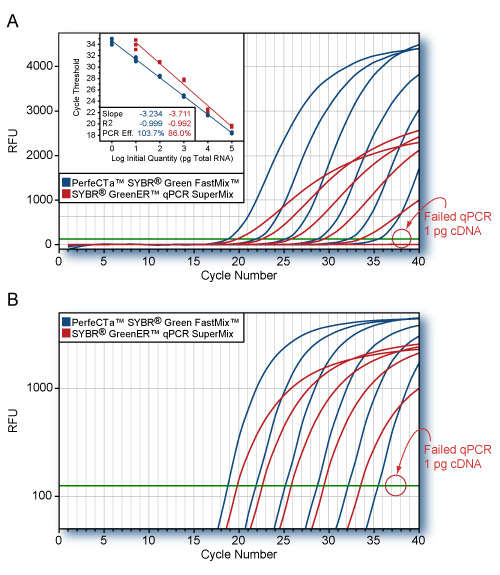
- 4X concentrated master mix allows greater flexibility with dilute samples – as little as 1 pg of total RNA
- Multiplexing capable with up to 5 targets
- Tough-tested for applications where PCR inhibition from template impurities may be a concern, such as: crude extracts, clinical specimens, or environmental samples.
- Temperature stabilized master mix enables convenient reaction setup at ambient room temperature
UltraPlex 1-Step ToughMix is intended for molecular biology applications. This product is not intended for the diagnosis, prevention or treatment of a disease.
Description
UltraPlex 1-Step ToughMix is a ready-to-use, 4X concentrated master mix 1-step reverse transcription and real-time quantitative PCR (RT-qPCR) of RNA templates using probe-based detection methods. First-strand cDNA synthesis and PCR amplification are carried out in the same tube without opening between steps. Optimized to deliver highly sensitive quantification of RNA viruses or low abundance RNA targets in uni- or highly multiplexed RNA detection assays, this reagent chemistry is optimized to deliver maximum assay sensitivity, precision and reproducibility with miniaturized reaction volumes and either conventional or accelerated thermal cycling protocols. UltraPlex 1-Step ToughMix contains all required components for RT-qPCR except RNA template and probe and is compatible with all dual-labeled probe chemistries.
UltraPlex 1-Step ToughMix (Low ROX/ ROX) (4X), contains:
- 4X reaction buffer containing optimized titrations of qPCR-grade dATP, dCTP, dGTP, dTTP, and magnesium.
- Ultra-pure recombinant proteins: qScript XLT reverse transcriptase, RNase inhibitor protein, and AccuStart II hot-start Taq DNA polymerase.
- Proprietary stabilizers additives and optimized ROX passive reference dye (if applicable).
Store components in a constant temperature freezer at -25°C to -15°C protected from light upon receipt. For lot specific expiry date, refer to package label, Certificate of Analysis or Product Specification Form.
Repeated freezing and thawing does not affect RT-qPCR performance.
- Applied Biosystems 5700
- Applied Biosystems 7000
- Applied Biosystems 7300
- Applied Biosystems 7700
- Applied Biosystems 7900
- Applied Biosystems 7900HT
- Applied Biosystems 7900 HT Fast
- Applied Biosystems StepOne™
- Applied Biosystems StepOnePlus™
- Applied Biosystems 7500
- Applied Biosystems 7500 Fast
- Stratagene Mx3000P®
- Stratagene Mx3005P™
- Stratagene Mx4000™
- Applied Biosystems ViiA 7
- Applied Biosystems QuantStudio™
- Agilent AriaMx
- Douglas Scientific IntelliQube®
- Quantabio Q
- BioRad CFX
- Roche LightCycler 480
- QIAGEN Rotor-Gene Q
- Other
- BioRad iCycler iQ™
- BioRad MyiQ™
- BioRad iQ™5
Product Flyers
Product Manuals
Publications
Benjamin Lee, Vaccine – 2020
ABSTRACT
Background Oral, live-attenuated rotavirus vaccines suffer from impaired immunogenicity and efficacy in low-income countries. Increasing the inoculum of vaccine might improve vaccine response, but this approach has been inadequately explored in low-income countries. Methods We performed a double-blind, parallel group, randomized controlled trial from June 2017 through June 2018 in the urban Mirpur slum of Dhaka, Bangladesh to compare vaccine take (primary outcome) among healthy infants randomized to receive either the standard dose or double the standard dose of oral Rotarix (GlaxoSmithKline) vaccine at 6 and 10 weeks of life. Infants with congenital malformations, birth or enrollment weight <2000 gm, known immunocompromising condition, enrollment in another vaccine trial, or other household member enrolled in the study were excluded. Infants were randomized using random permuted blocks. Vaccine take was defined as detection of post-vaccination fecal vaccine shedding by real-time reverse transcription polymerase chain reaction with sequence confirmation or plasma rotavirus-specific immunoglobulin A (RV-IgA) seroconversion 4 weeks following the second dose. Results 220 infants were enrolled and randomized (110 per group). 97 standard-dose and 92 high-dose infants completed the study per-protocol. For the primary outcome, no significant difference was observed between groups: vaccine take occurred in 62 (67%) high-dose infants versus 69 (71%) standard-dose infants (RR 0.92, 95% CI 0.67–1.24). However, in post-hoc analysis, children with confirmed vaccine replication had significantly increased RV-IgA responses, independent of the intervention. No significant adverse events related to study participation were detected. Conclusions Administration of double the standard dose of an oral, live-attenuated rotavirus vaccine (Rotarix) did not improve vaccine take among infants in urban Dhaka, Bangladesh. However, improved immunogenicity in children with vaccine replication irrespective of initial inoculum provides further evidence for the need to promote in-host replication and improved gut health to improve oral vaccine response in low-income settings. ClinicalTrials.gov: NCT02992197.
Benjamin Lee, Vaccine – 2020
ABSTRACT
Background:Oral, live-attenuated rotavirus vaccines suffer from impaired immunogenicity and efficacyin low-income countries. Increasing the inoculum of vaccine might improve vaccine response, but thisapproach has been inadequately explored in low-income countries.Methods:We performed a double-blind, parallel group, randomized controlled trial from June 2017through June 2018 in the urban Mirpur slum of Dhaka, Bangladesh to compare vaccine take (primary out-come) among healthy infants randomized to receive either the standard dose or double the standard doseof oral Rotarix (GlaxoSmithKline) vaccine at 6 and 10 weeks of life. Infants with congenital malforma-tions, birth or enrollment weight <2000 gm, known immunocompromising condition, enrollment inanother vaccine trial, or other household member enrolled in the study were excluded. Infants were ran-domized using random permuted blocks. Vaccine take was defined as detection of post-vaccination fecalvaccine shedding by real-time reverse transcription polymerase chain reaction with sequence confirma-tion or plasma rotavirus-specific immunoglobulin A (RV-IgA) seroconversion 4 weeks following the sec-ond dose.Results:220 infants were enrolled and randomized (110 per group). 97 standard-dose and 92 high-doseinfants completed the study per-protocol. For the primary outcome, no significant difference wasobserved between groups: vaccine take occurred in 62 (67%) high-dose infants versus 69 (71%)standard-dose infants (RR 0.92, 95% CI 0.67–1.24). However, in post-hoc analysis, children with con-firmed vaccine replication had significantly increased RV-IgA responses, independent of the intervention.No significant adverse events related to study participation were detected.Conclusions:Administration of double the standard dose of an oral, live-attenuated rotavirus vaccine(Rotarix) did not improve vaccine take among infants in urban Dhaka, Bangladesh. However, improvedimmunogenicity in children with vaccine replication irrespective of initial inoculum provides further evi-dence for the need to promote in-host replication and improved gut health to improve oral vaccineresponse in low-income settings.
Marleen Botermans, BioRxiv – 2020
ABSTRACT
Potato spindle tuber viroid and other pospiviroids can cause serious diseases in potato and tomato crops. Consequently, pospiviroids are regulated in several countries. Since seed transmission is considered as a pathway for the introduction and spread of pospiviroids, some countries demand for the testing of seed lots of solanaceous crops for the presence of pospiviroids. A real-time RT-PCR test, named PospiSense, was developed for testing pepper (Capsicum annuum) and tomato (Solanum lycopersicum) seeds for seven pospiviroid species known to occur naturally in these crops. The test consists of two multiplex reactions running in parallel, PospiSense 1 and PospiSense 2, that target Citrus exocortis viroid (CEVd), Columnea latent viroid (CLVd), pepper chat fruit viroid (PCFVd), potato spindle tuber viroid (PSTVd), tomato apical stunt viroid (TASVd), tomato chlorotic dwarf viroid (TCDVd) and tomato planta macho viroid (TPMVd, including the former Mexican papita viroid). Dahlia latent viroid (DLVd) is used as an internal isolation control. Validation of the test showed that for both pepper and tomato seeds the current requirements of a routine screening test are fulfilled, i.e. the ability to detect one infested seed in a sample of c.1000 seeds for each of these seven pospiviroids. Additionally, the Pospisense test performed well in an inter-laboratory comparison, which included two routine seed-testing laboratories, and as such provides a relatively easy alternative to the currently used tests.
Safety Data Sheets (SDS)
CofA (PSF)
PSF-95166-500-Lot#021890
PSF-95166-500-Lot#021891
PSF-95166-500-Lot#022969
PSF-95167-100-Lot#022422
PSF-95167-500-Lot#022423
PSF-95168-500-Lot# 022416
PSF-95168-100-Lot#023967
PSF-95168-500-Lot#023968
PSF-95166-500-Lot#024085
PSF-95168-500-Lot#024522
PSF-95168-500-Lot#024893
PSF-95166-100-Lot#025390
PSF-95166-500-Lot#025391
PSF-95168-500-Lot#025386
PSF-95167-100-Lot#025466
PSF-95168-500-Lot#025801
PSF-95166-100-Lot#026369
PSF-95166-500-Lot#026370
PSF-95168-100-Lot#026975
PSF-95168-100-Lot#027045
PSF-95166-100-Lot#027588
PSF-95166-500-Lot#027589
PSF-95168-500-Lot#027906
PSF-95166-500-Lot#028185
PSF-95166-100-Lot# 028736
PSF-95166-500-Lot#028737
PSF-95168-500-Lot#028877
PSF-95168-100-Lot#029530
PSF-95168-500-Lot#029531
PSF-95166-01K-Lot#029948
PSF-95167-100-Lot#029689
PSF-95167-100-Lot#030000
PSF-95167-500-Lot#030001
PSF-95166-100-Lot#030094
PSF-95168-100-Lot#030046
PSF-95166-500-Lot#030294
PSF-95167-500-Lot#030312
PSF-95166-01K-LOT#66139974
PSF-95168-500-LOT#66139972
PSF-95166-100-LOT#66140414
PSF-95166-500-Lot#028737
PSF-95166-100-LOT#66142496
PSF-95168-100-LOT#66141489
PSF-95168-500-LOT#66141490
PSF-95166-01K-LOT#66147370
PSF-95166-500-LOT#66148043
PSF-95166-500-LOT#66148043
PSF-95166-01K-LOT#66148967
PSF-95166-01K-LOT#66152573
PSF-95166-500-LOT#66153258
PSF-95168-100-LOT#66153260
PSF-95166-100-LOT#66154306
PSF-95168-500-LOT#66154460
PSF-95166-100-LOT#66155755
PSF-95166-500-LOT#66155754
PSF-95167-100-LOT#66155757
PSF-95168-100-LOT#66155955
PSF-95168-500-LOT#66155760
PSF-95166-01K -LOT#66157161
PSF-95166-500-LOT#66157160
PSF-95168-100-LOT#66157825
PSF-95168-500-LOT#66157129
PSF-95166-100-LOT#66158195
PSF-95166-500 Lot# 66158194
PSF-95167-100 Lot66158250